



Highly efficient and stable oxygen evolution reaction (OER) electrocatalysts are critical to the performance of anion exchange membrane water electrolysis (AEM-WE) devices. However, at ampere-level high current densities, the accumulation and retention of oxygen bubbles lead to localized mass-transfer limitations, blockage of active sites, and stress concentration at the electrode interface, significantly degrading catalytic efficiency and device lifetime. Achieving efficient mass transport and long-term stability under high current operation remains a key scientific challenge for green hydrogen technologies.
Recently, Prof. Licheng Sun’s team at the Center of Artificial Photosynthesis and Solar Fuels, Westlake University, reported a breakthrough in OER electrocatalysis. Their work, entitled “Post-Selenium-Leaching Induced Fast Micro-Bubble Detachment on Nickel-Iron-Based OER Catalyst for Efficient AEM-WE,” was published in Angewandte Chemie International Edition. This study reveals the deep mechanistic role of selenium (Se) modification and post-leaching effects in regulating microbubble behavior and interfacial mass transfer, providing a new materials strategy for achieving efficient and stable water electrolysis at high current densities.
Prof. Licheng Sun, Chair Professor at Westlake University and Director of the Center of Artificial Photosynthesis and Solar Fuels, is the corresponding author. Shiwen Ding, a PhD student at Westlake University/Zhejiang University, and Zhiheng Li, an assistant researcher at Westlake University, are co-first authors.
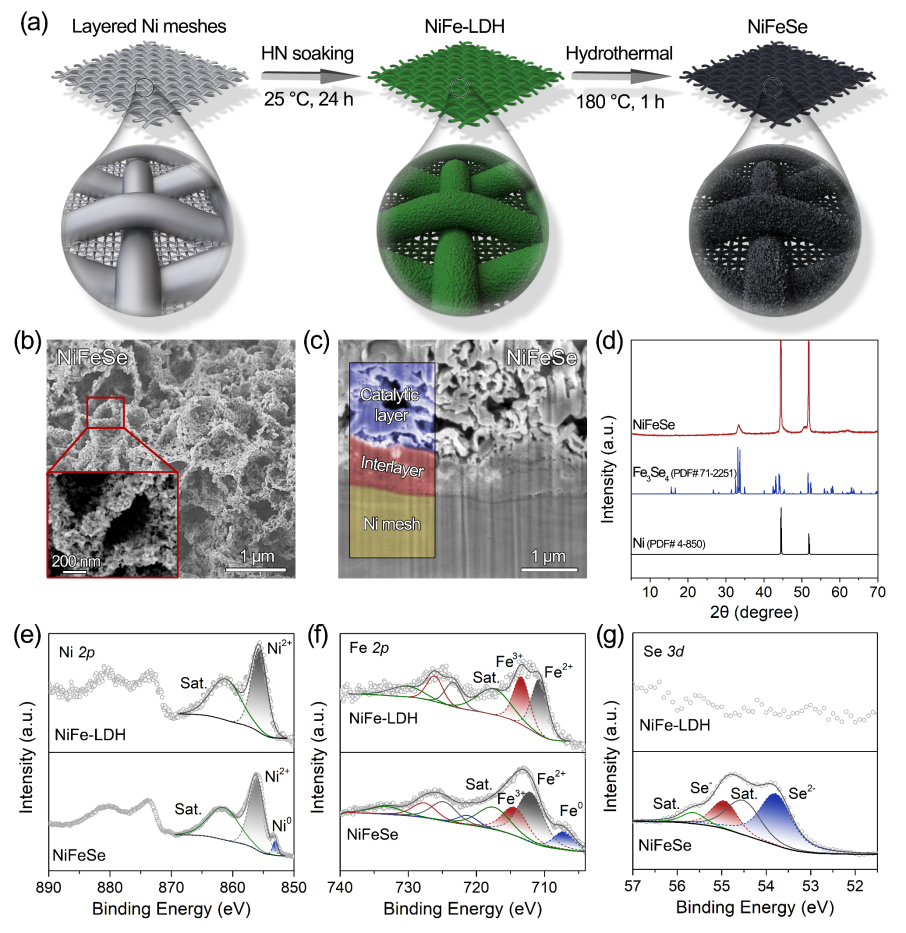
Figure 1. Synthesis and structural characterization of the NiFeSe catalyst
Starting from nickel–iron layered double hydroxide (NiFe-LDH), the research team constructed a NiFeSe catalyst with a rough surface and robust structure via a two-step heterogeneous nucleation–soaking followed by selenization modification strategy. The introduction of selenium not only enhances surface corrugation and porosity at the nanoscale, but also induces a post-leaching effect during the OER process, creating a superhydrophilic, low-adhesion interface. This interface effectively promotes the rapid generation and detachment of oxygen microbubbles.
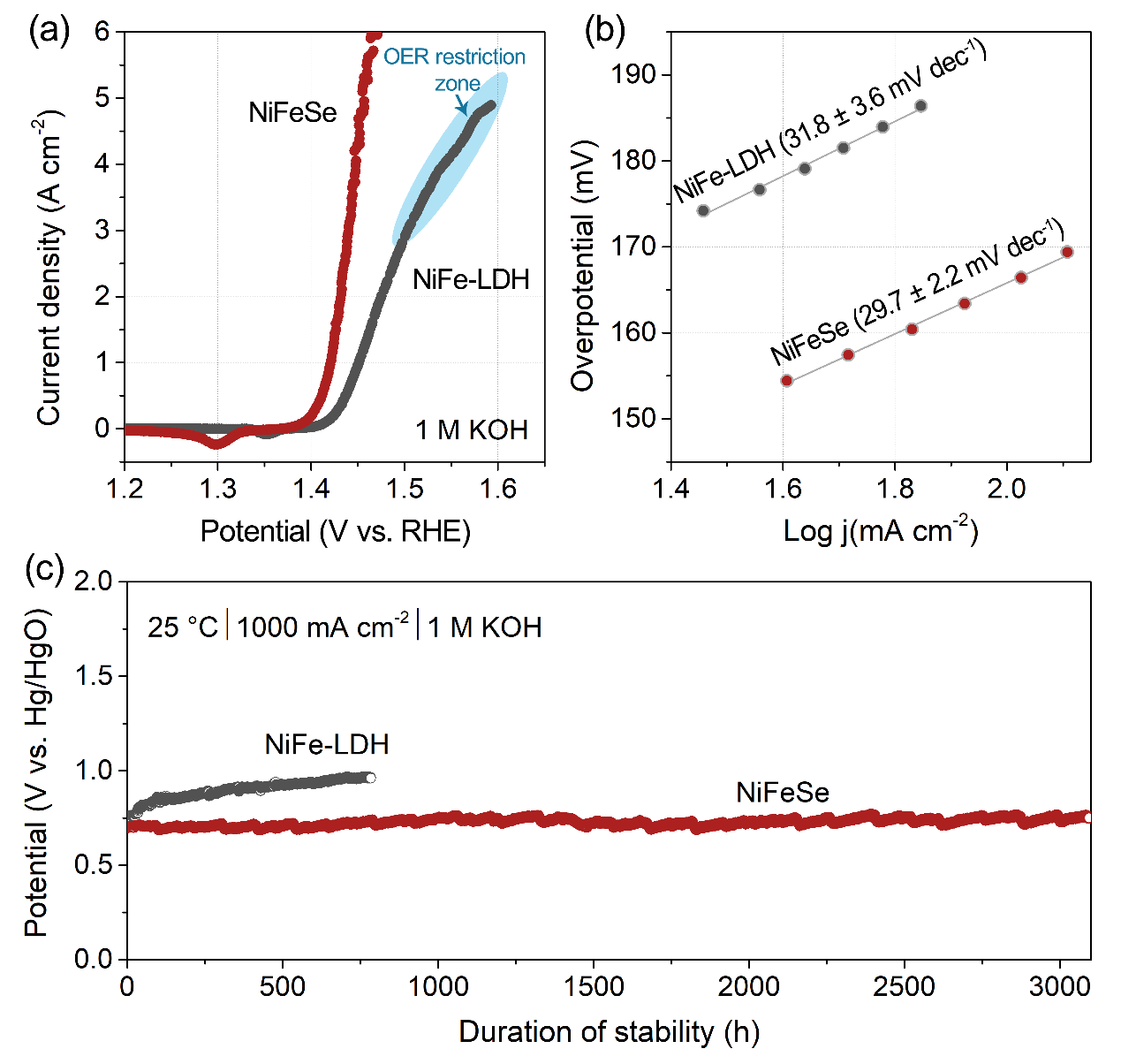
Figure 2. Comparison of electrochemical activity and stability of NiFeSe and NiFe-LDH for the oxygen evolution reaction (OER)
NiFeSe exhibits outstanding electrocatalytic performance in 1 M KOH, delivering an overpotential of only 190 mV at a current density of 1000 mA cm⁻2 and maintaining stable operation for over 3000 hours without noticeable degradation. Compared with conventional NiFe-LDH, NiFeSe shows a higher electrochemically active surface area (ECSA) and lower charge-transfer resistance, enabling more efficient charge transport and gas release.
Microbubble Dynamics Engineering Significantly Enhances Device Performance
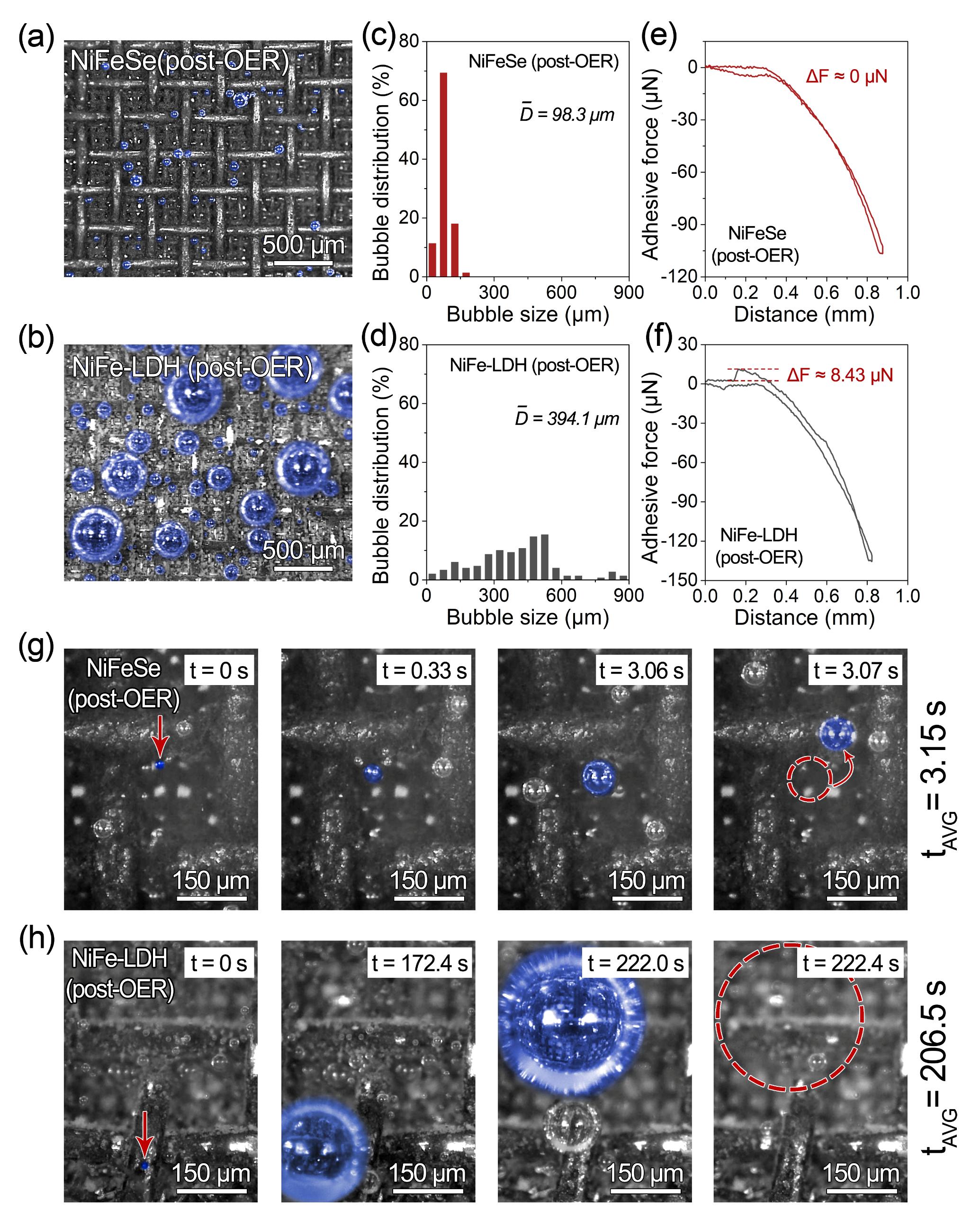
Figure 3. Pronounced differences in oxygen bubble generation and detachment between NiFeSe and NiFe-LDH
High-speed microscopic imaging reveals that oxygen bubbles formed on NiFeSe have an average diameter of ~98 µm and complete a full growth–detachment cycle within ~3.15 s. In contrast, bubbles on NiFe-LDH exceed 390 µm in diameter and require over 200 s to detach. This rapid-detachment behavior of NiFeSe markedly suppresses bubble blockage, accelerates interfacial renewal, and enhances solution mass transport.
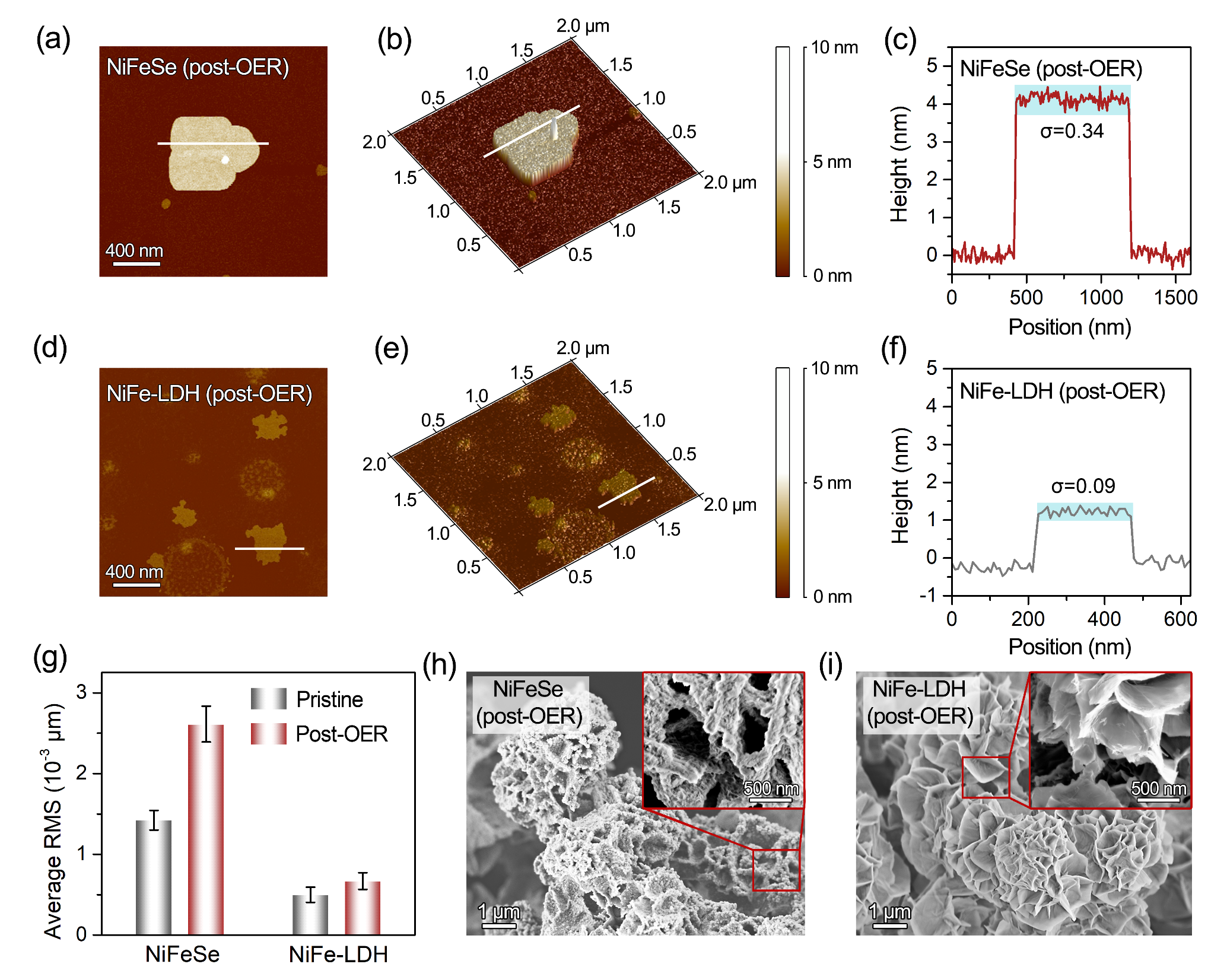
Figure 4. Surface morphology and roughness evolution of NiFeSe and NiFe-LDH before and after the oxygen evolution reaction (OER)
Atomic force microscopy (AFM) and contact-angle measurements show that, after OER, the surface roughness of NiFeSe increases by approximately fourfold. These results indicate superior hydrophilicity and bubble-repelling capability. The synergistic effect of increased roughness and high wettability endows NiFeSe with excellent bubble management and structural stability during OER.
Excellent Performance in Practical AEM Water Electrolysis (AEM-WE) Applications

Figure 5. Performance of the NiFeSe anode in practical anion exchange membrane water electrolysis (AEM-WE) devices
In practical AEM-WE tests, an electrolyzer assembled with a NiFeSe anode and a MoNi₄/MoO₂ cathode delivers an ultrahigh current density of 8800 mA cm⁻2 at 2.0 V and 80 °C, significantly exceeding the U.S. Department of Energy (DOE) 2026 water electrolysis target. The device also operates stably for over 2000 hours at 1000 mA cm⁻2. When scaled up to a 25 cm2 electrode area, the system maintains a strong output of 2.83 A cm⁻2 at 1.80 V and 60 °C, confirming the scalability and industrial potential of this approach.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Zhejiang Provincial Key R&D Program. The authors acknowledge partial support from the Research Center of Industrial Frontiers (RCIF) at Westlake University. We also thank the Instrumentation and Service Center for Physical Sciences (ISCPS), the Instrumentation and Service Center for Molecular Sciences (ISCMS), and the High-Performance Computing Center (HPC Center) at Westlake University for computational support, as well as the Micro/Nano Fabrication Center at Westlake University for equipment access and technical assistance. The authors are grateful to Dr. Husi Leng, Dr. Wen Wu, Dr. Jian Du, Dr. Wenlong Li, Dr. Yilong Zhao, Wentao Zheng, and Guoheng Ding for valuable discussions and assistance throughout this work.
Paper Information
Post‐Selenium‐Leaching Induced Fast Micro‐Bubble Detachment on Nickel‐Iron‐Based OER Catalyst for Efficient AEM‐WE.
Angew. Chem. Int. Ed., 2025, e202517132. DOI: 10.1002/anie.202517132
This research was supported by the National Key R&D Program of China (2022YFA0911900), the National Natural Science Foundation of China (22088102), the Zhejiang Provincial Key R&D Program (2024SSYS0063), and the Research Center of Industrial Frontiers at Westlake University
 |