Recently, Dr. Tao Wang from the Center of Artificial Photosynthesis for Solar Fuels at Westlake University proposed a groundbreaking theoretical study in collaboration with Dr. Frank Abild-Pedersen from the SUNCAT Center for Interface Science and Catalysis at SLAC National Accelerator Laboratory in the United States, which has been published in the prestigious journal Proceedings of the National Academy of Sciences (PNAS). This work introduces a novel catalyst design strategy with the potential to drastically reduce the enormous energy footprint of the global ammonia industry.
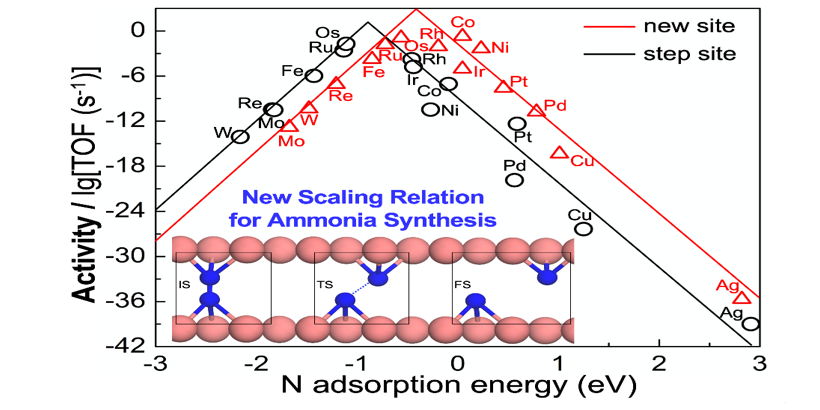
Although the century-old Haber-Bosch process sustains global agriculture through fertilizer production, it consumes 1-2% of the world's energy due to its reliance on extreme heat and pressure. To tackle this issue, a "confined dual-site" catalyst was proposed theoretically in our research. By using advanced computational models, the authors demonstrate that this unique structure can break traditional scaling relations in catalysis, allowing nitrogen molecules to be activated and converted much more efficiently. The study predicts that such a catalyst could achieve ammonia synthesis rates two to three orders of magnitude (100 to 1000 times) higher than the best-existing commercial catalysts under the same even much milder near-ambient conditions, offering a revolutionary pathway toward low-energy chemical manufacturing.