



Hydrogen Evolution Reaction (HER):
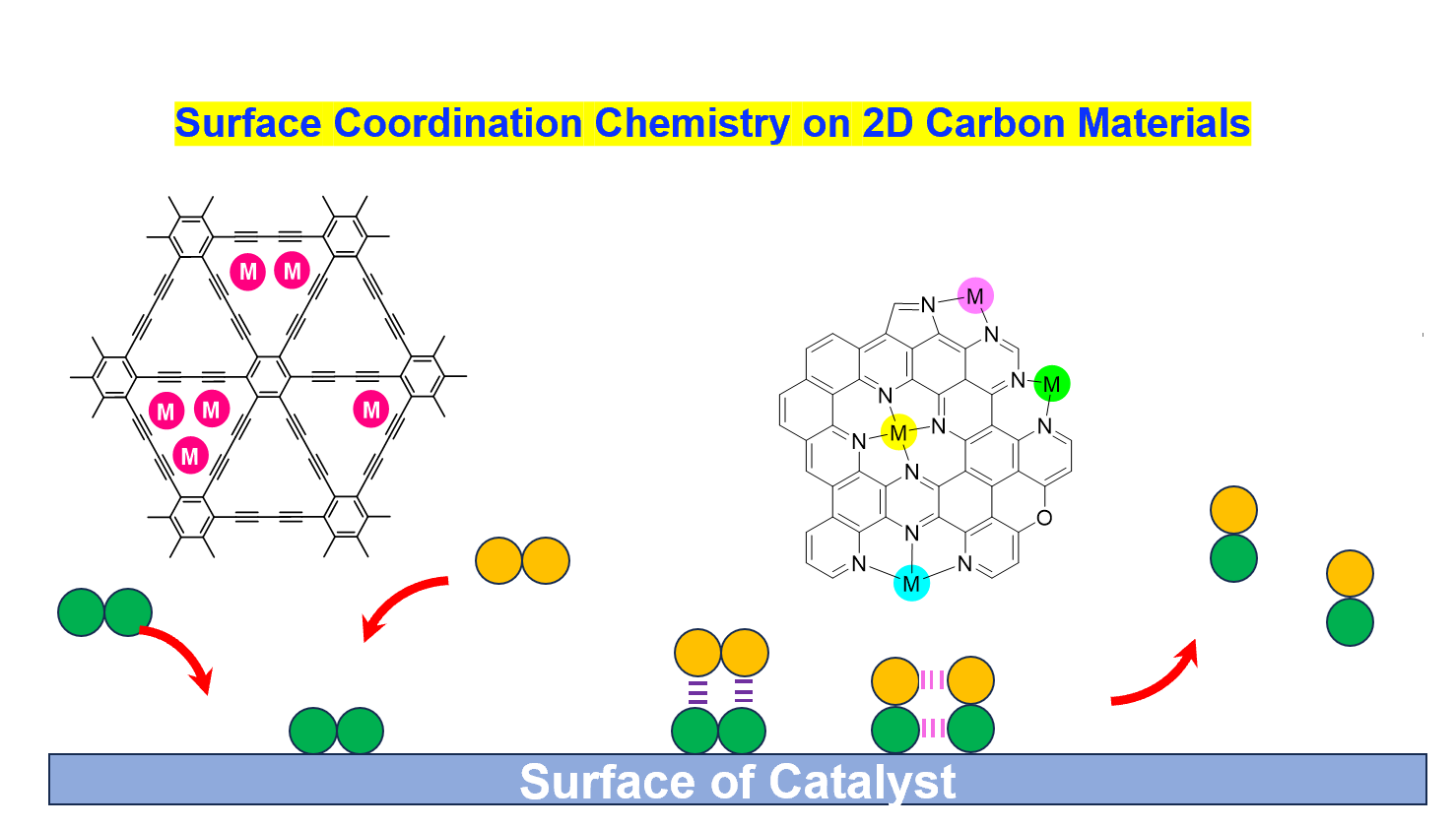
Heterogeneous hydrogen evolution catalysts generally offer advantages such as high activity, excellent stability, and simple electrode fabrication, but they suffer from limited structural tunability. To address this challenge, we propose to employ a surface coordination chemistry strategy on two-dimensional carbon materials. By utilizing graphdiyne, graphyne, and nitrogen-doped carbon materials as supports, transition metal atoms will be anchored via coordination interactions to construct hydrogen evolution catalysts with well-defined first and second coordination shells. Our research will focus on investigating how the structure of the coordination shells regulates the mechanism, catalytic activity, and stability.
Anion-Exchange Membrane (AEM):
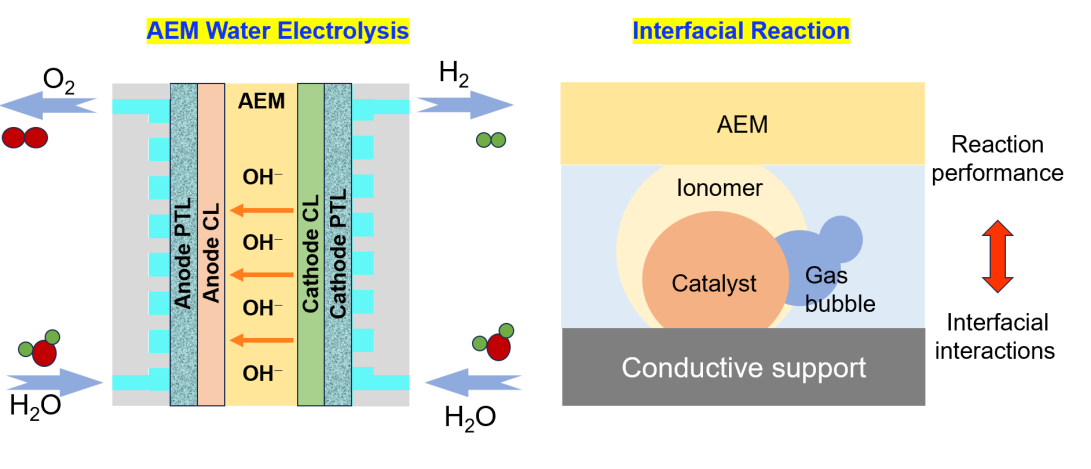
Ion exchange membrane water electrolysis technology employs dense anion exchange membranes as separators, establishing an alkaline interfacial environment between the cathode and anode. This approach combines the advantages of non-precious metal catalysts from alkaline water electrolyzers with the efficient gas-liquid separation characteristics of proton exchange membrane technology. Through rational structural design, we will develop anion exchange membrane materials with independent intellectual property rights, aiming at addressing critical scientific challenges such as low ion conductivity, insufficient operational stability, and optimization of multiphase interfaces.
Publication:
1.Liu, H.; Zou, H.; Wang, D.; Wang, C.; Li, F.; Dai, H.; Song, T.; Wang, M.; Ji, Y.; Duan, L.*, Second Sphere Effects Promote Formic Acid Dehydrogenation by a Single-Atom Gold Catalyst Supported on Amino-Substituted Graphdiyne. Angew. Chem. Int. Ed. 2023, e202216739. (https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202216739)
2. Zou, H.; Zhao, G.; Dai, H.; Dong, H.; Luo, W.; Wang, L.; Lu, Z.; Luo, Y.; Zhang, G.*; Duan, L.* Electronic perturbation of Cu single-atom CO2 reduction catalysts in a molecular way. Angew. Chem. Int. Ed. 2022, e202217220. (https://onlinelibrary.wiley.com/doi/10.1002/anie.202217220)
3.Rong, W.; Zou, H.; Tan, S.; Hu, E.; Li, F.; Tang, C.; Dai, H.; Wei, S.; Ji, Y.*; Duan, L.* Few-atom copper catalyst for the electrochemical reduction of CO to acetate: synergetic catalysis between neighboring Cu atoms. CCS Chem. 2022, doi:10.31635/ccschem.022.202201910. (https://www.chinesechemsoc.org/doi/10.31635/ccschem.022.202201910)
4.Rong, W.; Zou, H.; Zang, W.; Xi, S.; Wei, S.; Long, B.; Hu, J.; Ji, Y.; Duan, L.* Size-dependent activity and selectivity of atomic-level copper nanoclusters during CO/CO2 electroreduction. Angew. Chem. Int. Ed. 2021, 60, 466-472. (https://onlinelibrary.wiley.com/doi/10.1002/anie.202011836)
5.Zou, H.; Rong, W.; Wei, S.; Ji, Y.*; Duan, L.* Regulating kinetics and thermodynamics of electrochemical nitrogen reduction with metal single-atom catalysts in a pressurized electrolyser. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 29462-29468. (https://www.pnas.org/doi/10.1073/pnas.2015108117)
Update:Sept. 2025