



Research Field 1: Key Materials of Water Electrolysis for Hydrogen Production
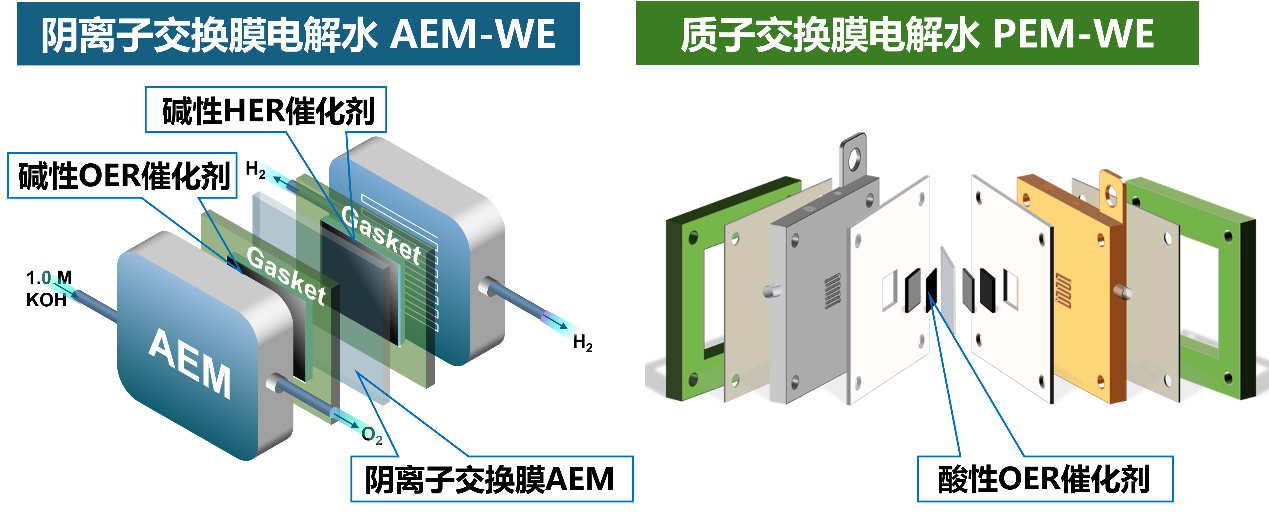
Water electrolysis is a core technology for green hydrogen production, and the electrolyzer used in this process is a complex integrated system involving various functional materials. Our research focuses on the investigation and development of key materials required for water electrolyzers. Based on molecular science, coordination chemistry, and materials chemistry, we design and synthesize a new generation of high-performance anion exchange membranes (AEMs) and ion-solvating membranes (ISMs). In addition, we explore simple and scalable preparation methods for alkaline oxygen evolution (OER) and hydrogen evolution (HER) catalysts, as well as develop low-iridium or iridium-free acidic OER catalysts for proton exchange membrane water electrolysis applications.
Publications:
1.X. Cui, Y. Ding, F. Zhang, X. Cao, Y. Guo, L. Sun, B. Zhang*, Reserved Charges in a Long-Lived NiOOH Phase Drive Catalytic Water Oxidation. Nature Chemistry, 2025, in press
2.Z. Wei, Y. Ding*, W. Shi, F. Zhang, Y. Song, X. Cui, Y. Guo, L. Sun, Q. Jiang, B. Zhang*, Lanthanum-Assisted Lattice Anchoring of Iridium in Co3O4 for Efficient Oxygen Evolution Reaction in Low-Iridium Water Electrolysis. Nature Communications, 2025, 16, 8145. DOI: 10.1038/s41467-025-63577-x.
3.Y. Song, W. Zhao, Z. Wang, W. Shi, F. Zhang, Z. Wei, X. Cui, Y. Zhu, T. Wang, L. Sun, B. Zhang, Sub‑4 nm Ru-RuO2 Schottky Nanojunction as a Catalyst for Durable Acidic Water Oxidation. Journal of the American Chemical Society, 2025, 147, 13775–13783. DOI: 10.1021/jacs.5c01876
4.B. Zhang, L. Fan, R. B. Ambre, T. Liu, Q. Meng, B. J. J. Timmer, and Licheng Sun*, Advancing Proton Exchange Membrane Electrolyzers with Molecular Catalysts, Joule, 2020, 4, 1408–1444. DOI: 10.1016/j.joule.2020.06.001.
5.X. Cui, T. Tang, F. Zhang, L. Sun, B. Zhang*, New Benchmark for Pure Nickel-Based Oxygen-Evolution Electrocatalyst: Tailored Large NiMoO4.xH2O Monocrystals for Complete Reconstruction. Applied Catalysis B: Environment and Energy, 2025, 366, 125024. DOI:10.1016/j.apcatb.2025.125024
Research Field 2: Electrocatalytic CO2 Reduction to Formformic Acid
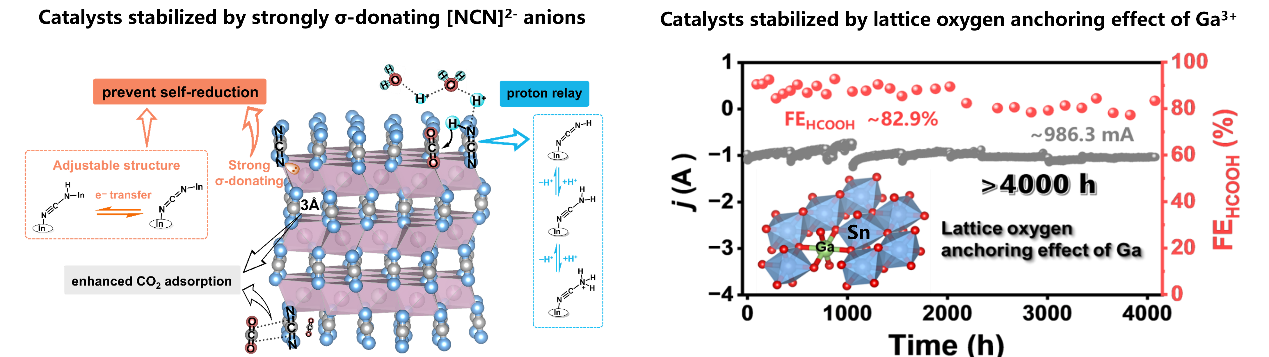
Formic acid is the most electron-economical product of CO₂ reduction, because it can serve both as a fuel for direct use in formic acid fuel cells and a hydrogen storage medium. In our lab, we introduce the concept of proton shuttling in catalysts. By investigating the tautomerism of the [NCN] structure in cyanamide compounds to facilitate proton transfer, the reaction kinetics are accelerated and the oxidation state stability of metal active centers can be maintained. Furthermore, through optimization of crystal and microstructures, we construct an acid-stable electrocatalytic CO₂ reduction catalyst system, ultimately developing an industrially promising electrocatalytic system for CO₂ reduction to formic acid.
Publications:
1.B. Jia, Z. Chen, K. Zhu, W. Shi, Z. Hu, T. Wang*, L. Sun, B. Zhang*, Gallium modulated tin oxide for continuous production of formic acid via durable acidic CO2 electroreduction, Science Advances, 2025, 11, eadw7326. DOI: 10.1126/sciadv.adw7326.
2.K. Zhu, B. Jia, Z. Chen, Z. Hu, L. Sun, T. Wang*, and B. Zhang*, Sn Catalysts with Build-in [NCN]2− as Proton Relay for Industrial-Grade CO2 Reduction at Low Overpotential, Angewandte Chemie International Edition, 2025, 137, e202507422. DOI: 10.1002/anie.202507422.
3.B. Jia, Z. Chen, C. Li, Z. Li, X. Zhou, T. Wang, W. Yang, L. Sun, and B. Zhang*, Indium Cyanamide for Industrial-Grade CO2 Electroreduction to Formic Acid, Journal of the American Chemical Society, 2023, 145, 14101−14111. DOI: 10.1021/jacs.3c04288.
Research Field 3: Electrocatalytic Ammonia Oxidation
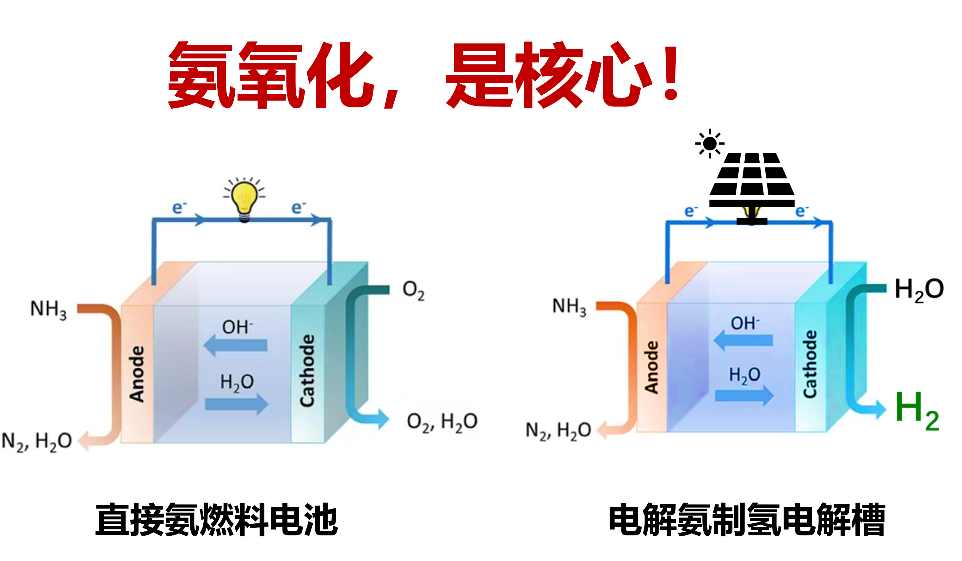
Although photovoltaic technology and water electrolyzers have enabled large-scale green hydrogen production, the large-scale storage of hydrogen remains a critical challenge. Converting green hydrogen into green ammonia offers an effective solution, as ammonia can be directly used as fuel in ammonia fuel cells or decomposed electrochemically to regenerate hydrogen. The two low-temperature utilization pathways including direct ammonia fuel cells and electrochemical ammonia cracking fundamentally rely on efficient electrochemical ammonia oxidation reactions (converting ammonia to nitrogen). Thus, achieving efficient ammonia‑to‑nitrogen conversion is the core bottleneck for the practical application of low‑temperature ammonia technologies. To address this challenge, our research will focus on: 1) rational molecular design for developing high‑performance ammonia oxidation catalysts; 2) elucidating fundamental reaction mechanisms; and 3) establishing clear structure‑activity relationships. These molecular‑level insights will guide the subsequent engineering design of heterogeneous catalysts for integration into ammonia fuel cells and electrolyzers.
Publications:
1.J. Li, X. Shi, F. Zhang, X. Lu, Y. Zhang, R.-Z. Liao*, B. Zhang *. Electrocatalytic Ammonia Oxidation by a Ruthenium Complex Bearing a 2,6-Pyridinedicarboxylate Ligand. JACS Au, 2025, 5 (4), 1812–1821. DOI: 10.1021/jacsau.5c00054.
2.H. Xiong, J. Yang, J. Li, Y. Cai, F. Zhang, J.-Y. Chen*, R.-Z. Liao, L. Sun, B. Zhang*. Electrochemical Ammonia Oxidation Catalyzed by a Ruthenium Complex with a Dangling Sulfonate Group. ACS Catalysis, 2025, 8633–8642. DOI: 10.1021/acscatal.5c01166.
3.J. Li, F. Zhang, H. Xiong, Y. Cai, B. Zhang*. Molecular Catalysts for Electrocatalytic Ammonia Oxidation. Science China Chemistry, 2024, 67, 3976−3993. DOI: 10.1007/s11426-024-2137-5.