



The Laboratory of Theoretical Catalysis and Intelligent Materials Design primarily focuses on key scientific challenges in anion exchange membrane water electrolysis for hydrogen production and related technologies (AEM-WE and beyond). It is dedicated to developing and applying multi-scale theoretical simulation methods and artificial intelligence techniques for the rational design of anion exchange membranes and high-performance catalysts, as well as deeply elucidating the complex catalytic reaction mechanisms and interaction dynamics at solid interfaces, ultimately providing critical theoretical insights for artificial photosynthesis in the production of solar fuels and high-value-added chemicals.
§ Multiscale Simulation and Intelligent Design of Anion Exchange Membrane (AEM)
Anion exchange membranes (AEMs) are one of the key components in AEM-WE technology. However, the lack of high-performance AEMs that meet industrial requirements has become a bottleneck hindering the advancement of AEM-WE technology. Therefore, developing AEMs with high stability, high conductivity, and robust mechanical strength is a core challenge for realizing the industrial application of AEM-WE technology. To address this, our laboratory has independently developed a kinetic trajectory analysis tool (RDAnalyzer), which has successfully enabled the decoupling and analysis of the complex hydroxide transport mechanisms within AEMs. This work provides a solid theoretical and practical foundation for the rational design of high-performance AEMs (as illustrated in Figure 1). Moving forward, we will further accelerate the research and development of high-performance AEMs by advancing high-precision multiscale theoretical simulation methods and artificial intelligence technologies.
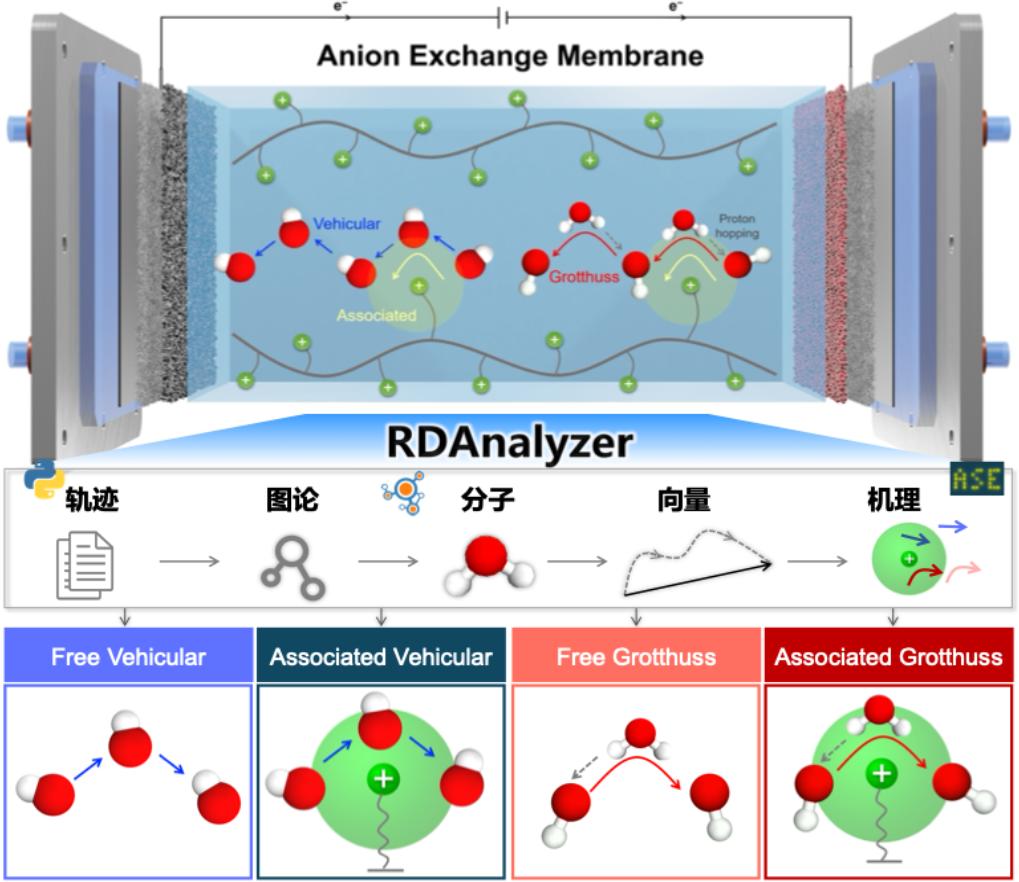
图1. 阴离子交换膜机理分析工具的开发与应用
§ AI-Driven Design of HER and OER catalysts
With the advancement of theoretical simulation methods and artificial intelligence technologies, the paradigm of theory-driven intelligent catalyst design is gradually becoming feasible and is expected to replace traditional "trial-and-error" catalyst design strategies. Currently, we have accumulated extensive experience in high-throughput computation and machine learning-driven intelligent catalyst design. Among our recent developments, an equivariant graph neural network (equivGNN) model, capable of analyzing chemical similarity in diverse complex catalytic systems, has enabled precise prediction of multiple key catalytic descriptors (Fig. 2A). Based on corresponding machine learning methods and our established stability-activity-cost screening framework, we accurately identified NiSb alloy with a double hexagonal close-packed (DHCP) crystal structure as the optimal HER catalyst (Fig. 2B). This catalyst has been successfully synthesized in the laboratory and applied in electrolyzers, demonstrating continuous stable operation for over 3,000 hours, with both stability and activity currently representing the highest level among non-precious HER catalysts. The research group will continue to advance the application of artificial intelligence in catalyst design for AEM-WE and beyond, accelerating the development of related catalysts.
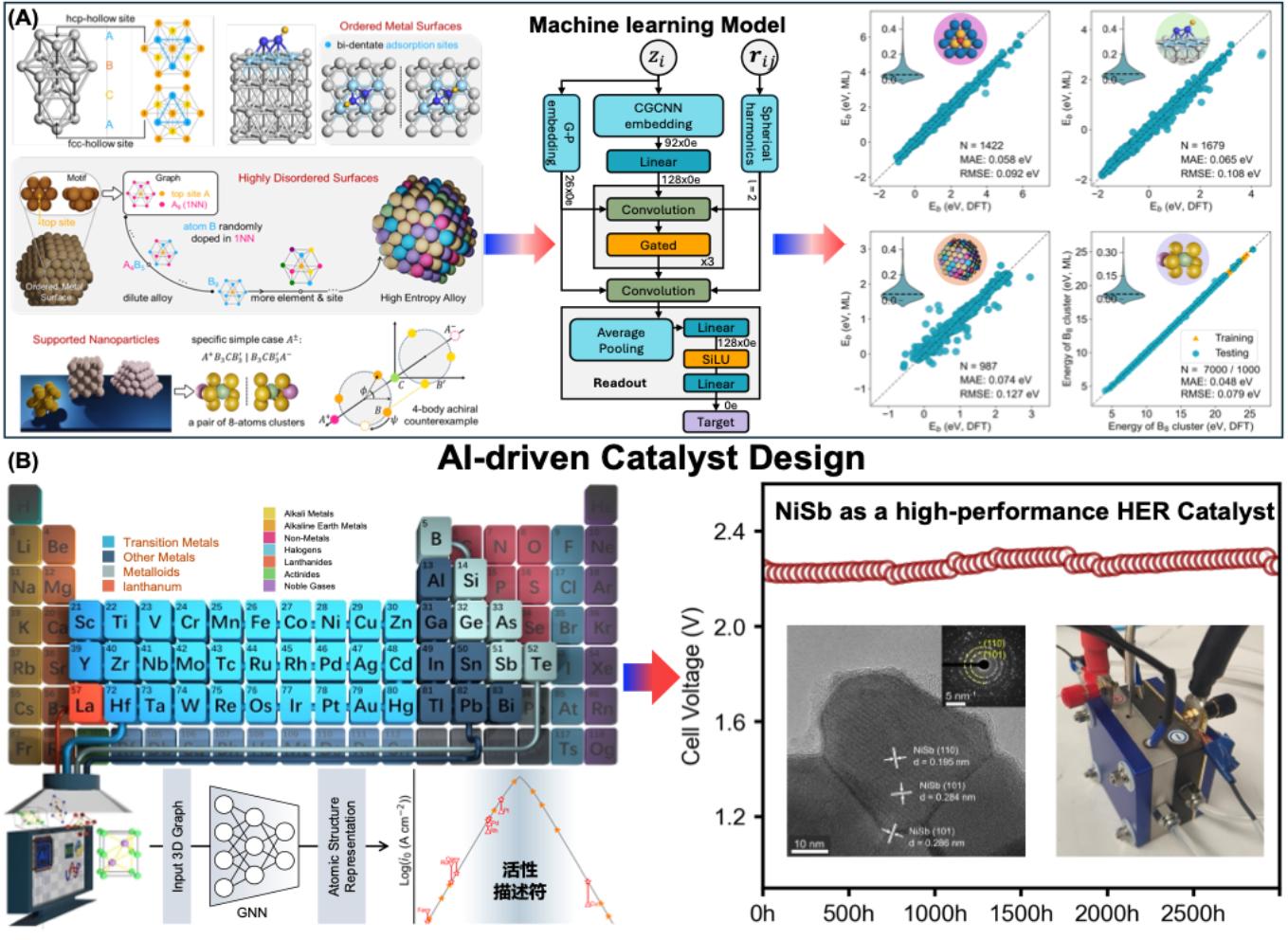
图2. AI驱动的水分解催化剂的设计筛选框架
§ Rational Design of Ammonia Synthesis Catalysts and Exploration of New Mechanisms
·Theoretical design of mild nitrogen-fixing catalysts
Ammonia (NH₃) is an indispensable chemical in agriculture and industry. However, the traditional Haber–Bosch process requires breaking the inert N≡N triple bond under high temperature and high pressure, leading to high energy consumption. Therefore, achieving efficient nitrogen conversion to ammonia under mild conditions is a shared goal for both academia and industry, yet it remains a significant challenge. Focusing on the key scientific issues in mild nitrogen fixation, we aim to efficiently activate the inert N≡N triple bond. Starting from theoretical exploration of novel catalytic mechanisms, we have proposed a confined dual-site nitrogen activation strategy and achieved low-barrier nitrogen activation by constructing a series of model catalysts (Figure 3A). Specifically, by using porous materials such as zeolites as supports and constructing dual-site catalysts with well-defined active sites and stable structures, we systematically investigate the influence of spatial configuration, types of metal active centers, and adsorption states of reactive species on the nitrogen fixation mechanism, catalytic activity, and selectivity. Ultimately, this provides an important theoretical reference for achieving efficient ammonia synthesis under mild conditions.
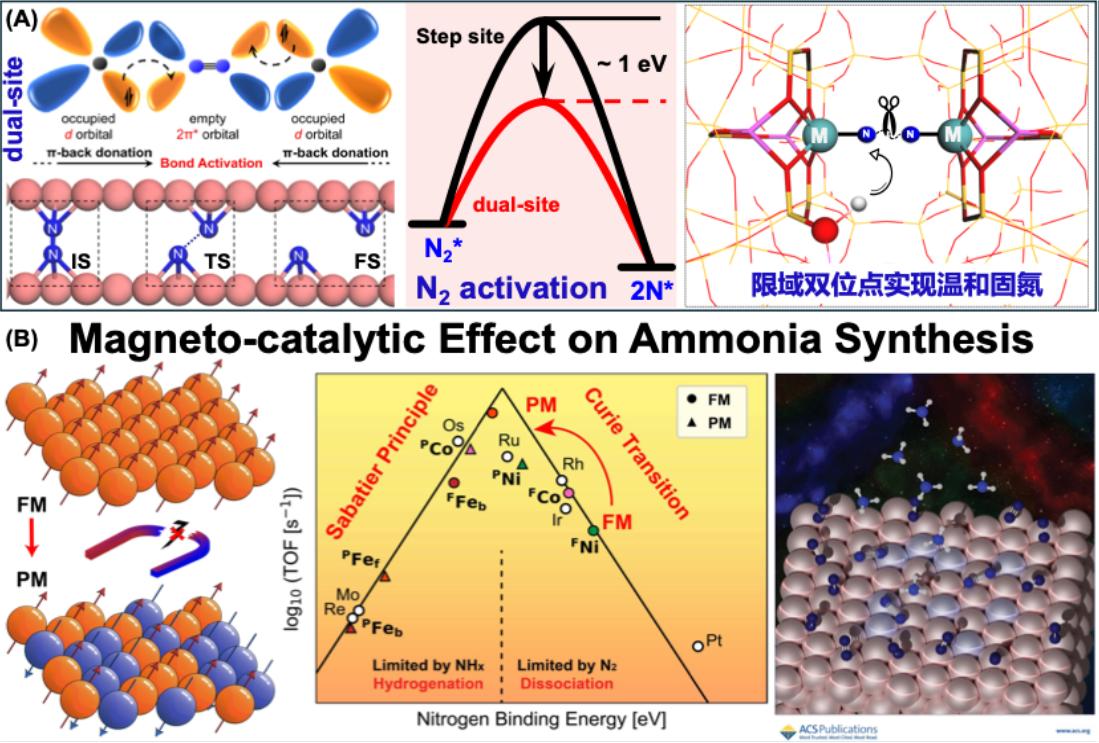
图3 温和固氮催化剂的理论设计和新机理探索
·The Mechanism of the Magnetic-Catalytic Effect on the Activity of Ammonia Synthesis
In addition to the theoretical design of catalysts for mild nitrogen fixation, we have successfully elucidated the impact of magnetic phase transitions on ammonia synthesis activity. Specifically, solid magnetic materials possess a unique physical property—the magnetic order-disorder phase transition at high temperatures. For instance, ferromagnets lose their spontaneous magnetization and become paramagnetic when the temperature exceeds the Curie point. Due to the influence of magnetic phase transitions, chemical reactions on ferromagnetic catalysts may exhibit different activities in different magnetic phases, a phenomenon known as the magneto-catalytic effect. Although this effect was firstly reported by the Swedish experimental scientist Professor Hedvall in the 1930s, the magneto-catalytic effect could not be thoroughly elucidated or widely applied at the time due to limitations in experimental characterization and theoretical computational methods, thus leading to delayed development in this field. Through theoretical calculations, we have revealed the mechanism by which changes in magnetic order influence catalytic performance in magnetic materials. Using ammonia synthesis as a model reaction, we found that the ferromagnetic-paramagnetic phase transition can enhance the ammonia synthesis activity of cobalt and nickel by 2–4 orders of magnitude. Moreover, cobalt with 55% magnetization reduction exhibits near-Sabatier-optimal performance as an ammonia synthesis catalyst (Figure 3B). As the magneto-catalytic effect is a universal physical phenomenon in solid magnetic materials, it holds promise as a new breakthrough for the development of high-performance heterogeneous catalysts.
§ Development and Application of Multi-scale Dynamic Simulation Program
Since AEM-WE is a complex integrated system coupling multiple components and fields, our group has developed a computationally efficient and broadly applicable multi-scale kinetic simulation program (Figure 4) to comprehensively model the impact of cross-scale factors on catalytic performance. This tool has already been successfully applied to elucidate how micro-environmental factors and mass transport at catalytic surfaces and interfaces influence electrocatalytic performance across scales, providing critical technical support for theoretical research in electrocatalytic systems.
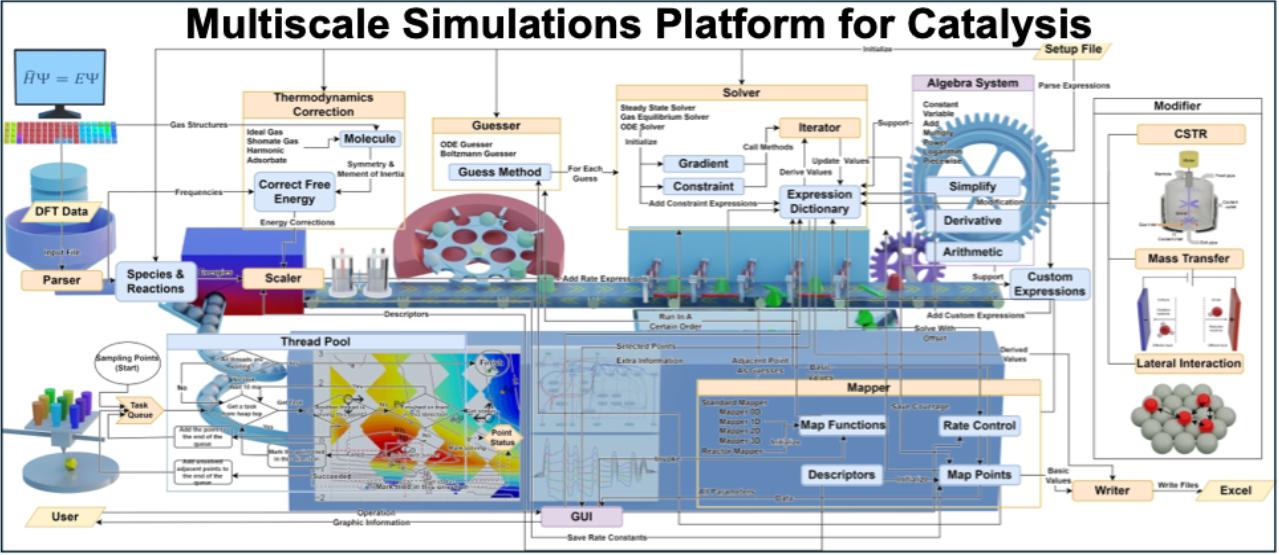
图4 多尺度动力学模拟程序
Publications:
1.Shou, W.; Zhao, W.; Liu Y.; Wang, T.*. Toward Rational Understanding of the Hydrogen Evolution Polarization Curves Through Multiscale Simulations. Nat. Commun., 2025, in press.
2.Cai, C.; Wang, T.*. Resolving chemical-motif similarity with enhanced atomic structure representations for accurately predicting descriptors at metallic interfaces. Nat. Commun., 2025, 16, 8761.(https://doi.org/10.1038/s41467-025-63860-x)
3.Han, C.; Liu, Y.; Wang, T.*. Structure–Activity Relation of the Oxide Path Mechanism for O–O Coupling on Rutile-Based Oxygen Evolution Reaction Catalysts. ACS Energy Lett. 2025, 10, 4511. (Highlighted by Nature Catalysis)(https://doi.org/10.1021/acsenergylett.5c01739)
4.Cai,C.; Lee,H.; Shi, W.; Liu, Y.; Zhang, B.*; Sun, L.; Wang, T.*. Nickel-Antimony Electrocatalyst for Durable Acidic Hydrogen Evolution Reaction in Proton Exchange Membrane Electrolyzers. ACS Energy Lett. 2025, 10, 1483.(https://doi.org/10.1021/acsenergylett.5c00233)
5.Ma, L.; Wang, T.*. Rational Understanding Hydroxide Diffusion Mechanism in Anion Exchange Membranes during Electrochemical Processes with RDAnalyzer. Angew. Chem. Int. Ed., 2024, e202403614.(https://doi.org/10.1002/anie.202403614)
6.Xu, G.; Sun, L.; Wang,T.*. Demagnetizing Ferromagnetic Catalysts to the Sabatier Optimal of Haber–Bosch Process. JACS Au 2024, 4, 1405.(https://doi.org/10.1021/jacsau.3c00785)
7.Liu, C.; Xu, G.; Wang, T.*. Theoretical Approach toward a Mild Condition Haber–Bosch Process on the Zeolite Catalyst with Confined Dual Active Sites. JACS Au 2023, 3, 3374.(https://pubs.acs.org/doi/10.1021/jacsau.3c00546)
8.Zhao, W.; Xu, G.; He, Z.; Cai, C.; Abild-Pedersen, F.; Wang, T.*. Toward carbon monoxide methanation at mild conditions on dual-site catalysts. J. Am. Chem. Soc. 2023, 145, 8726.(https://pubs.acs.org/doi/10.1021/jacs.3c02180)
9.Xu, G.; Cai, C.; Wang,T.*. Toward Sabatier Optimal for Ammonia Synthesis with Paramagnetic Phase of Ferromagnetic Transition Metal Catalysts. J. Am. Chem. Soc. 2022, 144, 23089.(https://doi.org/10.1021/jacs.2c10603)
10.Wang, T.*; Abild-Pedersen, F.*. Achieving industrial ammonia synthesis rates at near-ambient conditions through modified scaling relations on a confined dual site. Proc. Natl. Acad. Sci. 2021, 118, e2106527118.(https://doi.org/10.1073/pnas.2106527118)
11.Wang,T.*; Sha, J.; Sabbe, M.; Sautet, P.*; Pera-Titus, M.*; Michel, C.*. Identification of active catalysts for the acceptorless dehydrogenation of alcohols to carbonyls. Nat. Commun., 2021, 12, 5100.(https://doi.org/10.1038/s41467-021-25214-1)
12.Wang,T.; Li, G.; Cui, X.*; Abild-Pedersen, F.*. Identification of earth-abundant materials for selective dehydrogenation of light alkanes to olefins. Proc. Natl. Acad. Sci. 2021, 118, e2024666118.(https://doi.org/10.1073/pnas.2024666118)
13.Wang,T.; Ibañez, J.; Wang, K.; Fang, L.; Sabbe, M.; Michel, C.; Paul, S.; Pera-Titus, M.*; Sautet, P.*. Rational design of selective metal catalysts for alcohol amination with ammonia. Nature Catalysis, 2019, 2, 773.(https://doi.org/10.1038/s41929-019-0327-2)