



We are a "Spectroscopy for Catalysis" laboratory, focusing on in situ/operando spectroscopic characterization of electrocatalytic surfaces and interfaces, as well as the investigation of reaction mechanisms. We are committed to achieving two key goals: first, expanding the theoretical understanding in the fields of electrochemistry and electrocatalysis, advancing fundamental knowledge "onto the bookshelf"; second, conducting mechanism-guided design for critical applied catalytic systems, proposing feasible and effective strategies for performance enhancement, and facilitating the translation of catalytic technologies "onto the market."
Our primary research systems cover fundamental scientific challenges in electrocatalytic CO₂ reduction (CO₂RR), hydrogen evolution (HER), oxygen evolution (OER), as well as anion exchange membranes and water electrolysis technologies (AEM & AEM-WE). Research directions include:
(a) The Problem of Cross-Scale Catalytic Mechanism in Electrocatalytic CO2 Reduction
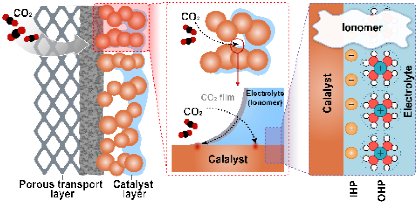
Electrocatalytic CO₂ reduction reaction (CO₂RR) can convert greenhouse gases into high-value fuels and chemicals, playing a crucial role in achieving the "dual-carbon" goals. Simultaneously, bond-forming processes involving "C–H," "C=O," and "C=C" bonds, with CO as a key intermediate, are among the most fundamental reactions at electrochemical interfaces. Therefore, a deep exploration of the reaction mechanisms in CO₂RR is not only essential for the efficient conversion of CO₂ but also holds significant guiding value for understanding broader surface and interfacial electrochemical processes. Focusing on CO₂RR mechanisms, we have conducted systematic in situ and operando spectroscopic studies. On one hand, we have made significant progress in understanding the stability and selectivity modulation mechanisms of copper-based catalysts by using classical spectroscopic techniques, particularly gaining insights into copper surface reconstruction-induced evolution of *CO configurations and their kinetic transformation processes. On the other hand, we continuously develop novel in situ and operando spectroscopic methods to advance research on pulsed electrochemical CO₂RR technologies and catalytic mechanisms of molecular modifications on membrane electrode surfaces, striving to achieve breakthroughs both methodologically and in mechanistic understanding.
Publications: J. Am. Chem. Soc. 2024, 146, 4242;J. Am. Chem. Soc. 2025, 147, 31497;J. Am. Chem. Soc. 2023, 145, 20655;J. Am. Chem. Soc. 2024, 146 , 23901;Proc. Natl. Acad. Sci. U.S.A. 2025, 122 , e2418144122.
(b) Study on the Structure-Effect Relationship and Transport Properties of Ion Exchange Membranes
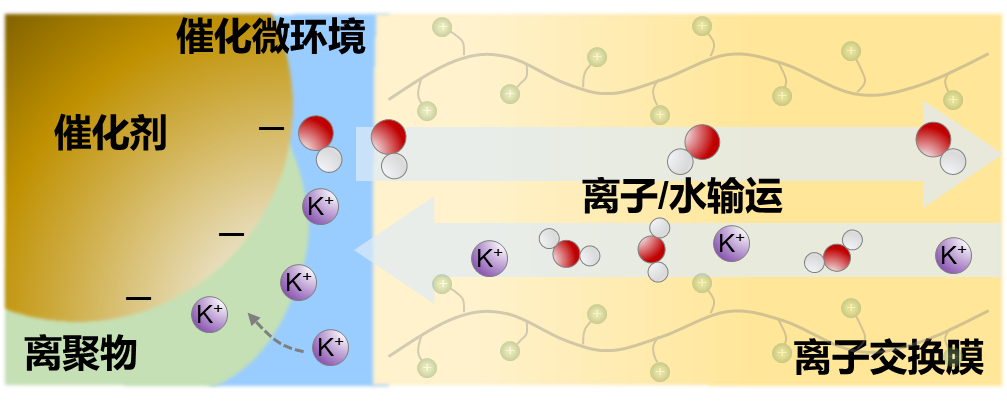
Ion-exchange membranes are critical components in energy electrochemical devices, as their ion and water transport properties directly regulate the formation of the catalytic microenvironment at the cathode, serving as a core determinant of overall device performance. To gain an in-depth understanding of the structure-property relationships and microscopic transport mechanisms of these membranes, we conduct systematic characterization and mechanistic studies from multiple dimensions, which include utilizing techniques such as atomic force microscopy (AFM) and small-angle X-ray scattering (SAXS) to analyze the nano- and mesoscale structures of membrane materials, thereby establishing correlations between microscopic morphology and performance. Through ion conductivity measurements and characterization of water diffusion behavior via nuclear magnetic resonance (NMR) and infrared spectroscopy (IR), we systematically reveal the mass transport properties and kinetic patterns of the membranes. Additionally, we develop in situ/operando characterization methods applicable to membrane electrode assembly (MEA) devices under real operating conditions, bridging mechanistic insights from the material to the device scale. These efforts are dedicated to clarifying the behavior and mechanisms of ion-exchange membranes in complex electrochemical environments, providing a scientific foundation for the rational design of high-performance membrane materials and devices.
Current research efforts remain ongoing.
(c) Research on the Fundamental Mechanisms of "Solid-Liquid" and "Solid-Gas-Liquid" Interface Electrocatalysis
The "solid-liquid" and "solid-gas-liquid" interfaces represent the most critical electrochemical energy device interfaces, and the fundamental electrochemical processes and catalytic mechanisms occurring in these surfaces play a decisive role in determining catalytic performance. To investigate the evolution patterns and catalytic regulation of these crucial interfaces, we continuously explore and develop novel electrochemical spectroscopic techniques. We aim to elucidate how surface catalysis is precisely regulated within a microenvironment filled with water, ions, and surface-adsorbed species. Particular emphasis is placed on the regulatory role of non-covalent interactions between interfacial species and their surrounding microenvironmental solutions on catalytic behavior.
Publications:Nat. Catal. 2025, 6, 1115;J. Am. Chem. Soc. 2025, 147, 10260;
J. Phys. Chem. C 2025, doi.org/10.1021/acs.jpcc.5c07614;